Medication transformed—a new approach to the molecule you know and trust

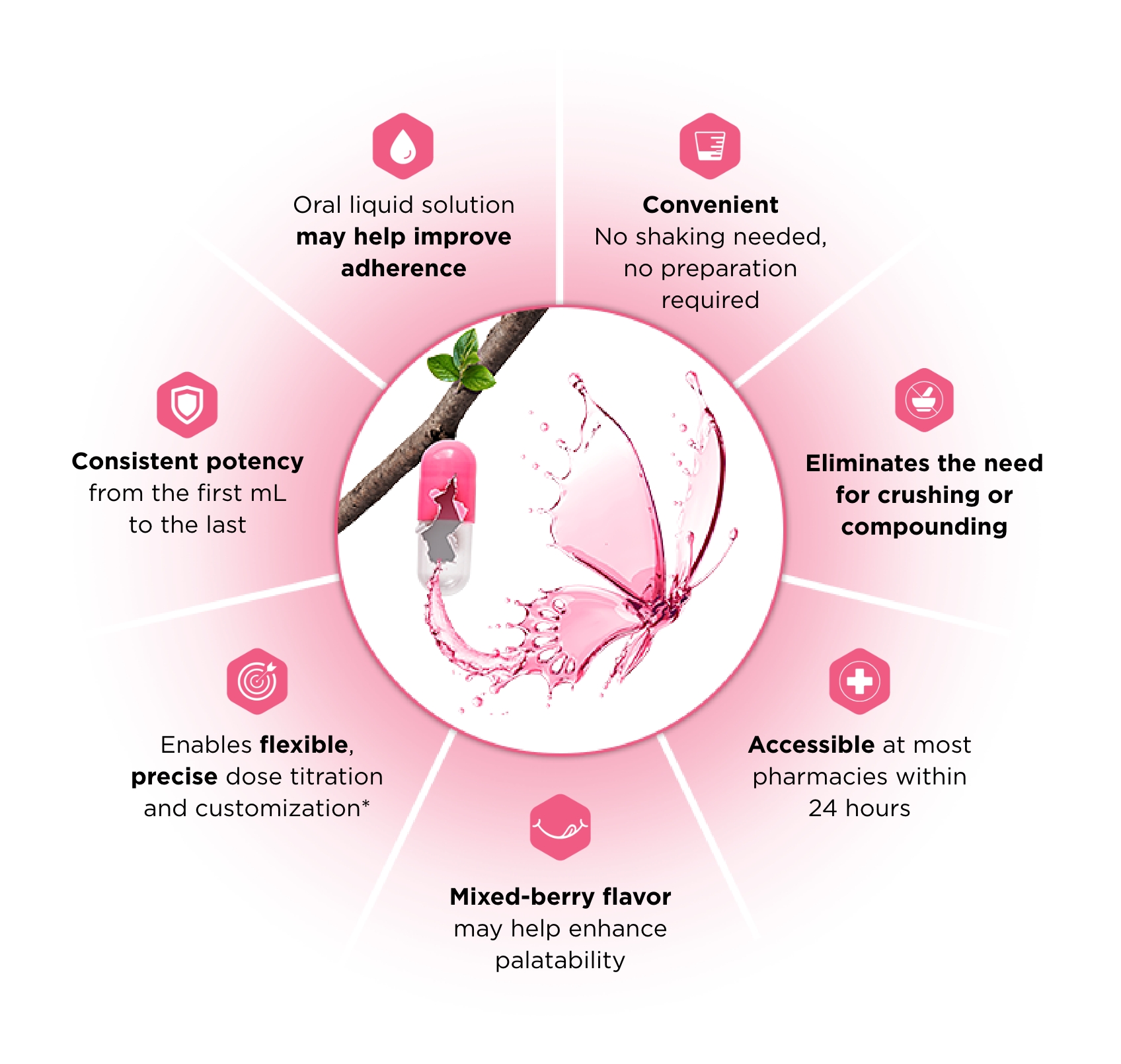
*A calibrated measuring device is recommended to measure and deliver the prescribed dose accurately. A household teaspoon or tablespoon is not an adequate measuring device. Once opened, unused portion should be discarded after 30 days.
Important Safety Information (cont)
Suicidal Behavior and Ideation: Antiepileptic drugs (AEDs), including EPRONTIA, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Cognitive/Neuropsychiatric Adverse Reactions: EPRONTIA can cause cognitive/neuropsychiatric adverse reactions. The most frequent adverse reactions can be classified into 3 categories: 1) cognitive-related dysfunction (confusion, difficulty with concentration, difficulty with memory, speech or language problems); 2) psychiatric/behavior disorders; 3) somnolence or fatigue.
Please see additional Important Safety Information throughout, and the accompanying full Prescribing Information.